Abstract
Background
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma subtype, being nodal involvement its main characteristic. However, data about Extranodal (EN) involvement is not reported in Latin American patients. Our aim was to evaluate the clinical features, treatment patterns outcomes of Peruvian patients with ENFL from two cancer centers and validate FLIPI1, FLIPI2, PRIMA and POD-24 prognostic index in our cohort.
Methods:
This is a retrospective study, including all patients with a pathological diagnosis of FL grade 1 to 3A treated at the National Institute of Neoplastic Diseases and Oncosalud, both in Lima, Peru from 2010 to 2019. All cases were reviewed by specialized pathologists. Baseline clinical and pathological data were collected. Responses were assessed based on the Lugano criteria. Overall survival (OS) was estimated using the Kaplan-Meier method. Differences were compared with the log-rank test.
Results
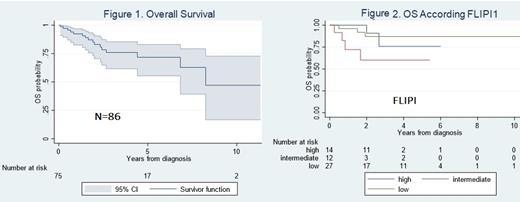
A total of 86 patients were evaluated. The median age was 61 years (30-91), 43% were male, 20% had bulky disease (≥6 cm in diameter), 51% had stage III/IV disease, 31% had hemoglobin <12 g/dl, 11% had serum albumin <3 g/dl, 25% had elevated serum LDH, 31% had B2-microglobulin ≥3,5 mg/l, 27% had bone marrow involvement and 19% had lymph node sites >4. The most frequent EN sites were gastrointestinal, bone marrow, cutaneous and breast with 22%, 21% and 9%, and 6%% respectively. Low, intermediate and high-risk FLIPI1 was seen in 56%, 23% and 21% of patients, respectively. Low, intermediate and high-risk FLIPI2 was seen in 28%, 58% and 14% of patients, respectively. Low, intermediate, and high PRIMA was seen in 57%, 10% and 33%, respectively. 55 patients (64%) received any treatment, 47% received CHOP ± rituximab (R), 16% CVP ± R, 22% radiotherapy alone, 9% CHOP, 15% other treatments. Response data were available in 44 patients with complete response in 45%, partial response in 43% and no response in 12%, for an overall response rate of 88%. From patients who received CHOP/CVP ± R, 13% patients had disease progression within 24 months of first treatment initiation (POD24). For the entire cohort (N=88) the median follow-up time was 2.1 years (interquartile range [IQR] 0.08-11.3), median overall survival was 8.25 years (IQR 4.4-not reached [NR]). 5y OS was71.7% (95% CI 55.3-82.9), figure 1. For the FLIPI group 5y OS for low, intermediate and high FLIPI were 86.9% (95% CI 64.7-95.6), 60.1% (95% CI 24.4-83.2) and 75.7% (95% CI 30.4-93.7), respectively (p=0.07; Figure 2). For the FLIPI2 group (N=58) 5y OS for low, intermediate and high FLIPI2 were 79.5% (95% CI 39.3-94.5), 77% (95% CI 55.3-89.2) and 86% (95% CI 33.4-97.8), respectively (p=0.86). For the PRIMA group (N=38) 5y OS for low, intermediate and high PRIMA was 86% (95%55.6-96.6), 100% (95% CI 100), and 80% (95% CI 42-95), respectively (p=0.80). Patients who had and did not have POD24 had median OS of NR (IQR 0.6-NR) and NR (IQR 2.69-NR), respectively. 5y OS for patients who had and did not have POD24 was 71.2 % (95% CI 48-85) and 75% (12.7-96), respectively (p<0.87).
Conclusion:
Peruvian patients with ENFL showed a higher rate of female patients. Gastrointestinal involvement was the most common primary site. The OS rates is similar to our nodal involvement cohort. Chemoimmunotherapy is the standard approach to FL patients, which is associated with high rates of overall response. In our study FLIPI, FLIPI2, PRIMA and POD24 were not predictors for OS, but larger cohorts and longer follow-up are needed to find more accurate predictors of survival in these patients.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal